Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Number of neutrons = rounded mass number − atomic number Atoms of the element chromium (Cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? Neutrons= mass number ʹ atomic number, 9-4=5;5 neutrons Practice: 1. Complete this table. Atomic Number Mass Number Number of protons Number of neutrons Number of electrons Symbol of Element 9 10 14 15 47 22 55 25 Br 8 16 47 61 16 16 Pb 2. What is the difference between mass number and atomic number 3. This two minute video shows how to read the periodic table. The terms 'atomic number' and 'atomic mass' are also defined. Find more free tutorials, videos.
How can atomic number and mass number be used to find the numbers of protons, electrons, and neutrons?
2 Answers
- Atomic number is the number of protons
- Atomic mass subtract atomic number is the number of neutrons
- Atomic number is the number of electrons in a neutral atom
Atomic Number And Mass Number
Explanation:

The atomic number is assigned based on the number of protons, so the atomic number is always the same as the number of protons
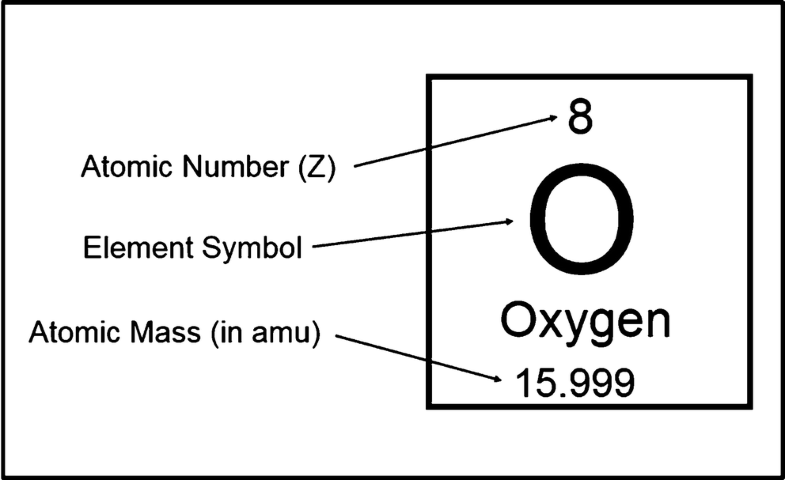
Atomic mass is the sum of protons and neutrons. Protons and neutrons both have masses of 1 amu, but the mass of electrons is negligible so it is left out. The atomic mass of an element is never a round number because it's a weighted average of all the isotopes of the element. Just round the number to the nearest integer get the most likely atomic mass.
In a neutral atom number of protons = number of electrons since there is no overall charge. In an ion, add an electron for every negative charge and subtract an electron for every positive charge (ex.
Ex.
Atomic Number And Mass Number Table
Hp officejet pro 8600 download for mac. The atomic number of O is 8 and the mass number is ~16
So it has Download terraform for mac.
- 8 protons
- 8 neutrons (16-8)
- 8 electrons (since we're talking about a neutral O atom)
Well the atomic number,
Explanation:
.....And for the neutral atom,

The
Note that
Related questions
