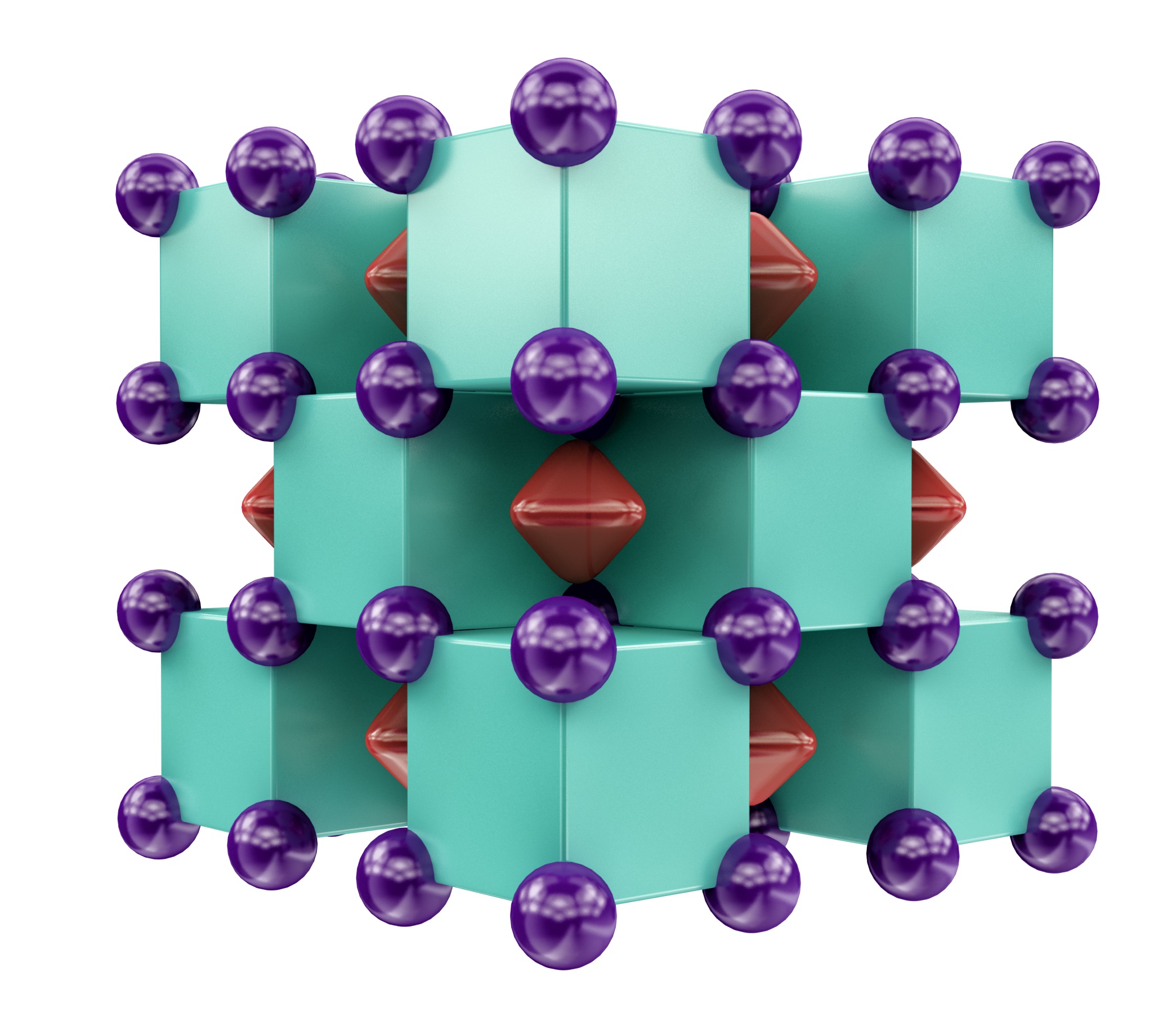
Molar mass of Na2He is ± 0.0000020 g/mol Convert between Na2He weight and moles. Initially, the Na2He compound was found to consist of Na8 cubes, of which half were occupied by helium atoms and half were empty. “Yet, when we performed chemical bonding analysis of these structures, we found each ‘empty’ cube actually contained an eight-center, two-electron bond,” Boldyrev says. Na2He: a stable compound of helium and sodium at high pressure. Imperial College London. Here's the crystal structure of Na2He - a solid formation of alternating sodium (purple) and helium (green) atoms, with electrons (red) shared in the voids between them: Artem R. Oganov 'It's not a real bond,' like ionic and covalent bonds, Popov told Gizmodo. 'But the helium does stabilise the structure.
A stable compound of helium and sodium at high pressure
Abstract
Helium is generally understood to be chemically inert and this is due to its extremely stable closed-shell electronic configuration, zero electron affinity and an unsurpassed ionization potential. It is not known to form thermodynamically stable compounds, except a few inclusion compounds. Here, using the ab initio evolutionary algorithm USPEX and subsequent high-pressure synthesis in a diamond anvil cell, we report the discovery of a thermodynamically stable compound of helium and sodium, Na2He, which has a fluorite-type structure and is stable at pressures >113 GPa. We show that the presence of He atoms causes strong electron localization and makes this material insulating. This phase is an electride, with electron pairs localized in interstices, forming eight-centre two-electron bonds within empty Na8 cubes. We also predict the existence of Na2HeO with a similar structure at pressures above 15 GPa.
Na2e-r1-9e0
- Condensed Matter - Materials Science


Disodium helide[1] (Na2He) is a compound of helium and sodium that is stable at high pressures above 113 gigapascals (1,130,000 bar). It was first predicted[2] using USPEX code and then synthesised in 2016.[3]
Synthesis[edit]
Na2 Age of empires 2 download for mac free. He was predicted to be thermodynamically stable over 160 GPa and dynamically stable over 100 GPa. This means it should be possible to form at the higher pressure and then decompress to 100 GPa, but below that it would decompose. Compared with other binary compounds of other elements and helium, it was predicted to be stable at the lowest pressure of any such combination. This also means, for example, that a helium-potassium compound is predicted to require much higher pressures of the order of terapascals.
The material was synthesized by putting tiny plates of sodium in a diamond anvil cell along with helium at 1600 bar and then compressing to 130 GPa and heating to 1,500 K with a laser.[3] Disodium helide is predicted to be an insulator and transparent.[3] At 200 GPa the sodium atoms have a Bader charge of +0.599, the helium charge is −0.174, and the two-electron spots are each near −0.511.[3] So this phase could be called disodium helium electride. Disodium helide melts at a high temperature near 1,500 K, much higher than the melting point of sodium. When decompressed, it can keep its form as low as 113 GPa.[3] As pressure increases, the sodium is predicted to gain more positive charge, the helium to lose negative charge and the free electron density to increase. Download anydesk for mac free. Energy is compensated by the relative shrinking of the helium atoms and the space for electrons.[4]
Structure[edit]
Na2hedp
Disodium helide has a cubic crystal structure, resembling that of fluorite. At 300 GPa the edge of a unit cell of the crystal has a = 3.95 Å. Each unit cell contains four helium atoms on the centre of the cube faces and corners, and eight sodium atoms at coordinates halfway between the center and each corner. Double electrons (2e−) are positioned on each edge and the centre of the unit cell.[note 1] Each pair of electrons is spin paired. The presence of these isolated electrons makes this an electride. The helium atoms do not participate in any bonding; however, the electron pairs can be considered as an eight-centre two-electron bond.
Footnotes[edit]
- ^Each face is shared by two cells, each edge is shared by four cells, and each corner is shared by eight cells.
Na2heo
References[edit]
- ^'Under Pressure, Helium Stops Being a Bystander'. insidescience.org. 2018-03-28. Retrieved 2020-11-14.
Then, in 2017, researchers synthesized a stable compound from helium and sodium known as disodium helide under the kinds of high pressures seen within gas giants, suggesting this compound might be found in nature and not just in labs.
- ^Saleh, Gabriele; Dong, Xiao; Oganov, Artem; Gatti, Carlo; Qian, Guang-rui; Zhu, Qiang; Zhou, Xiang-Feng; Wang, Hiu-tian (5 August 2014). 'Stable Compound of Helium and Sodium at High Pressure'. Acta Crystallographica Section A. 70 (a1): C617–C617. arXiv:1309.3827. doi:10.1107/S2053273314093826.
- ^ abcdeDong, Xiao; Oganov, Artem R.; Goncharov, Alexander F.; Stavrou, Elissaios; Lobanov, Sergey; Saleh, Gabriele; Qian, Guang-Rui; Zhu, Qiang; Gatti, Carlo; Deringer, Volker L.; Dronskowski, Richard; Zhou, Xiang-Feng; Prakapenka, Vitali B.; Konôpková, Zuzana; Popov, Ivan A.; Boldyrev, Alexander I.; Wang, Hui-Tian (6 February 2017). 'A stable compound of helium and sodium at high pressure'. Nature Chemistry. 9 (5): 440. arXiv:1309.3827. Bibcode:2017NatCh..9.440D. doi:10.1038/nchem.2716. PMID28430195.
- ^Wang, Hui-Tian; Boldyrev, Alexander I.; Popov, Ivan A.; Konôpková, Zuzana; Prakapenka, Vitali B.; Zhou, Xiang-Feng; Dronskowski, Richard; Deringer, Volker L.; Gatti, Carlo; Zhu, Qiang; Qian, Guang-Rui; Saleh, Gabriele; Lobanov, Sergey; Stavrou, Elissaios; Goncharov, Alexander F.; Oganov, Artem R.; Dong, Xiao (May 2017). 'A stable compound of helium and sodium at high pressure - Supplementary Information table 5'(PDF). Nature Chemistry. 9 (5): 440–445. Bibcode:2017NatCh..9.440D. doi:10.1038/nchem.2716. ISSN1755-4349. PMID28430195.
